Superfluidity in 4He
Cooling down a substance in the gaseous phase ordinarily leads to a phase transition to the liquid phase and then a subsequent transition to the solid phase. This is not the case with He4. It instead has two liquid phases; one of which displays strange, superfluid behaviour.
Why Helium Is Special

Helium is the only element that does not crystallise into solid form as its temperature approaches 0K under standard pressure. Quantum effects make the solid phase of matter thermodynamically unstable. As such, it has historically been used as the prototypical example of ultra-cold quantum fluids.
Superfluid Properties
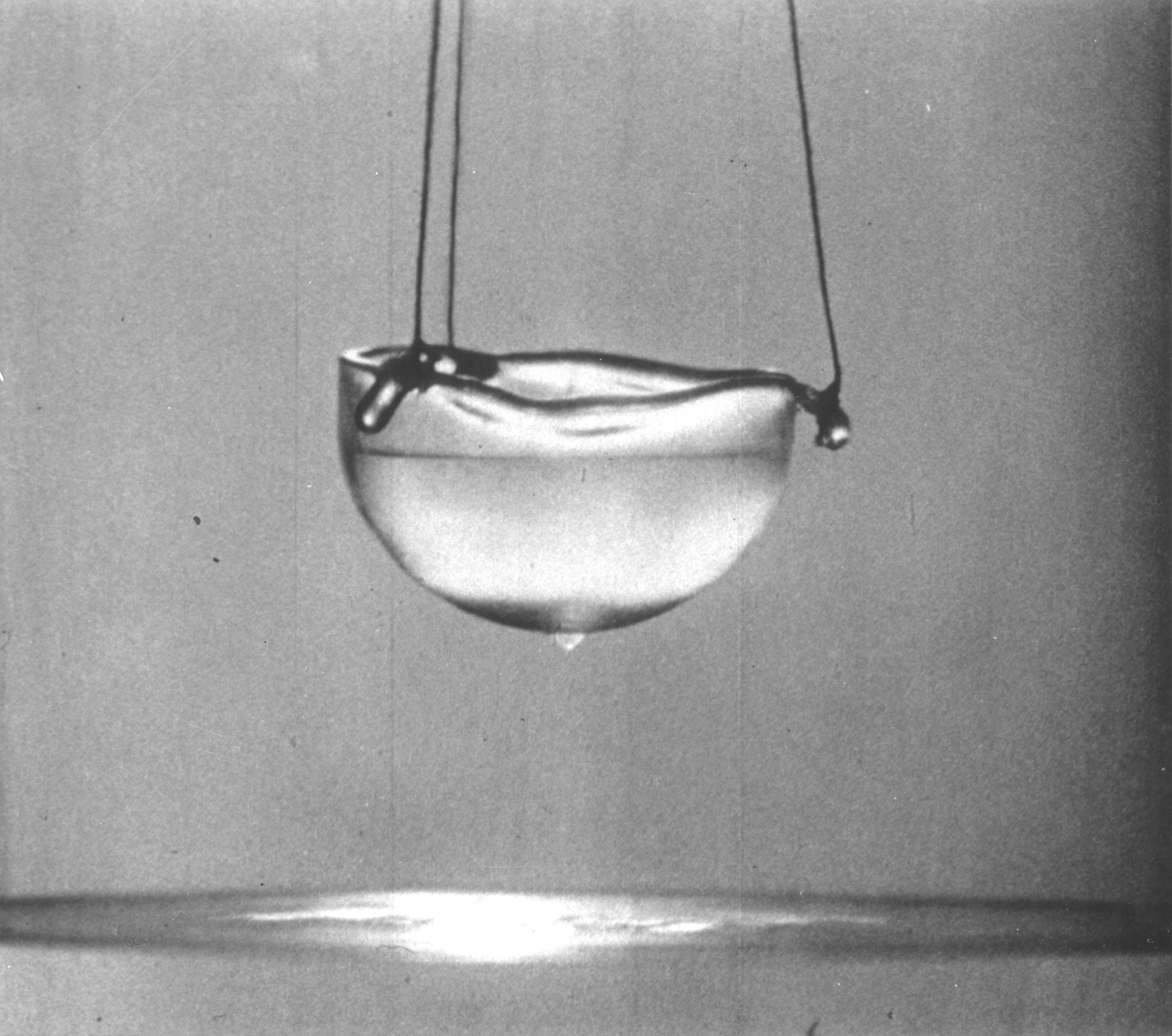
Superfluids behave in very peculiar ways. It can be shown that the dynamics of superfluids can be decomposed into superfluid and regular or "normal" fluid components. These two components have very different thermodynamic and mechanical properties which lead to some very unusual dynamical results.
Superfluid Vortices
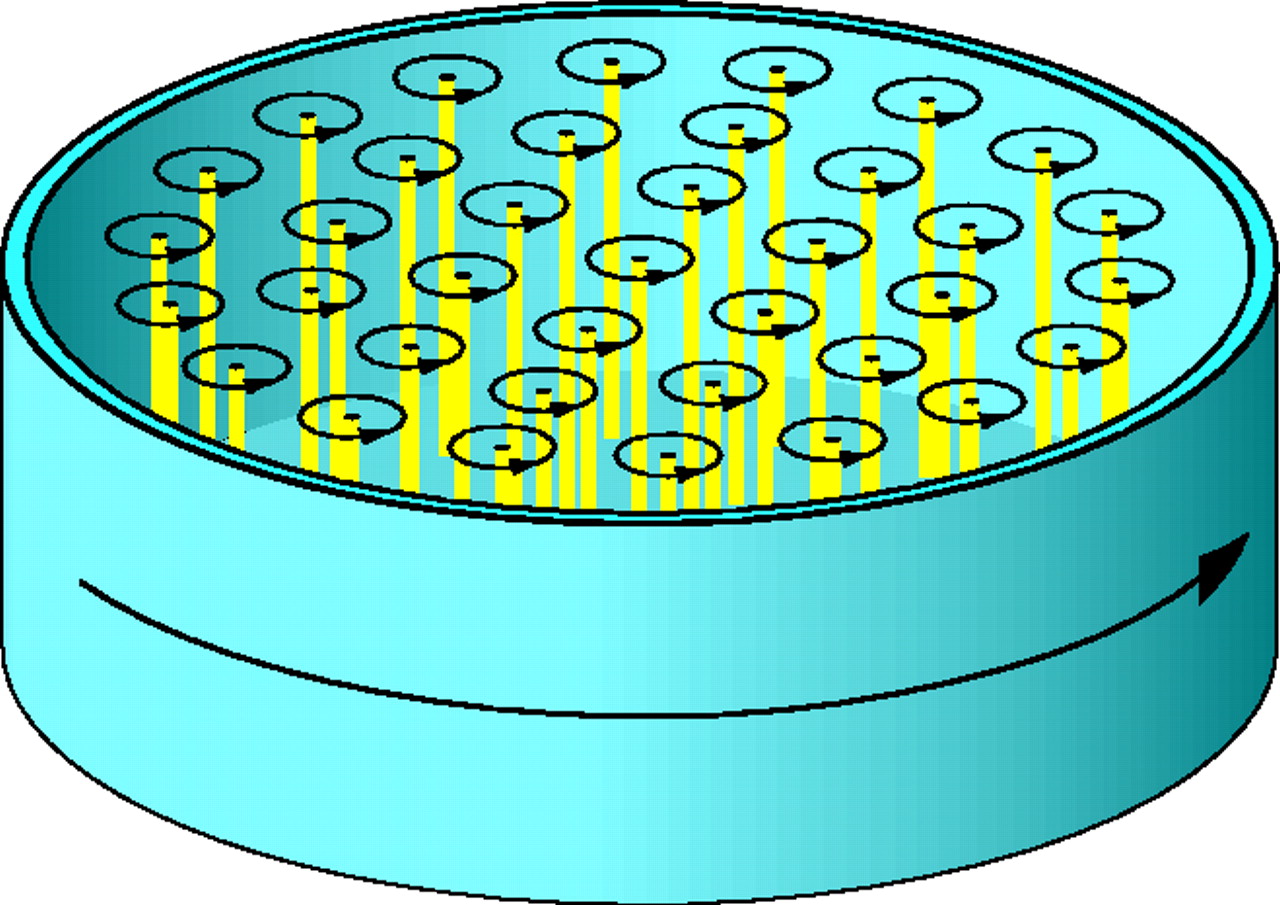
A point around which a fluid circulates is called a vortex. They may be familiar from regular fluids and yet they are also exhibited in a superfluid such as He 4. In a superfluid, the circulation due to vortices is quantised and this leads to discrete jumps in the rotational velocity of a superfluid.
Landau's Quasiparticles

What is the cause of superfluidity? It was not fully understood until Lev Davidovich Landau [1908-1968] undertook a thorough study of the relationship between the energy and the momentum of excitations inside the superfluids. These excitations were interpreted as two types of quasiparticles: phonons and rotons.
Resources
For further information on the subject of superfluidity and the related subject of Bose-Einstein condensates, the following are helpful.
Books
- Bose-Einstein Condensation by L. Pitaevskii and S. Stringari
- Superconductivity,Superfluids and Condensates by J. Annett
- Bose Condensed Gases At Finite Temperatures by A. Griffin, T. Nikuni and E. Zaremba